Description
Course Contents
- Ionic bonding, metallic bonding, covalent bonding and co-ordinate (dative covalent) bonding
- Shapes of simple molecules and bond angles
- Bond polarities and polarity of molecules
- Intermolecular forces, including hydrogen bonding
- Bond energies and bond lengths
- Lattice structure of solids
- Bonding and physical properties
Learning Outcomes
Candidates should be able to:
- (a) show understanding that all chemical bonds are electrostatic in nature and describe:
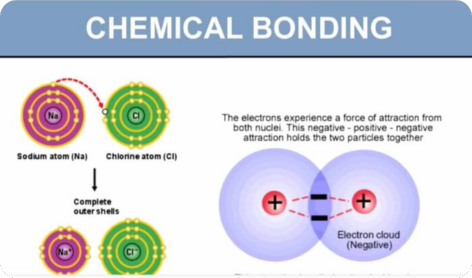
- (i) ionic bond as the electrostatic attraction between oppositely charged ions
- (ii) covalent bond as the electrostatic attraction between a shared pair of electrons and positively charged nuclei
- (iii) metallic bond as the electrostatic attraction between a lattice of positive ions and delocalised electrons
- (b) describe, including the use of ‘dot-and-cross’ diagrams,
- (i) ionic bonding as in sodium chloride and magnesium oxide
- (ii) covalent bonding as in hydrogen; oxygen; nitrogen; chlorine; hydrogen chloride; carbon dioxide; methane; ethene
- (iii) co-ordinate (dative covalent) bonding, as in formation of the ammonium ion and in the Al2 Cl6 molecule
- (c) describe covalent bonding in terms of orbital overlap (limited to s and p orbitals only), giving σ and π bonds (see also Section 11.1)
- (d) explain the shapes of, and bond angles in, molecules such as BF 3 (trigonal planar); CO 2 (linear); CH4 (tetrahedral); NH 3 (trigonal pyramidal); H2 O (bent); SF 6 (octahedral) by using the Valence Shell Electron Pair Repulsion theory
- (e) predict the shapes of, and bond angles in, molecules analogous to those specified in (d)
- (f) explain and deduce bond polarity using the concept of electronegativity (quantitative treatment of electronegativity is not required)
- (g) deduce the polarity of a molecule using bond polarity and its molecular shape (analogous to those specified in (d));
- (h) describe the following forces of attraction (electrostatic in nature):
- (i) intermolecular forces, based on permanent and induced dipoles, as in CHCl3 (l); Br2 (l) and the liquid noble gases
- (ii) hydrogen bonding, using ammonia and water as examples of molecules containing –NH and –OH groups
- (i) outline the importance of hydrogen bonding to the physical properties of substances, including ice and water
- (j) explain the terms bond energy and bond length for covalent bonds
- (k) compare the reactivities of covalent bonds in terms of bond energy, bond length and bond polarity
- (l) describe, in simple terms, the lattice structure of a crystalline solid which is:
- (i) ionic, as in sodium chloride and magnesium oxide
- (ii) simple molecular, as in iodine
- (iii) giant molecular, as in graphite and diamond
- (iv) hydrogen-bonded, as in ice
- (v) metallic, as in copper
(the concept of the ‘unit cell’ is not required) - (m) describe, interpret and/or predict the effect of different types of structure and bonding on the physical properties of substances
- (n) suggest the type of structure and bonding present in a substance from given information