Showing 1–6 of 17 results
-
Acid Base & Solubility Equilibria (Digital Notes)
$300.00 Add to cartSolubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
-

Alkanes (Digital Notes)
Read moreAlkanes are organic compounds that consist of single-bonded carbon and hydrogen atoms. The formula for Alkanes is CnH2n+2, subdivided into three groups – chain alkanes, cycloalkanes, and the branched alkanes.
-
Arenes (Digital Notes)
$100.00 Add to cartArenes are aromatic hydrocarbons. The term “aromatic” originally referred to their pleasant smells (e.g., from cinnamon bark, wintergreen leaves, vanilla beans and anise seeds), but now implies a particular sort of delocalized bonding. Aromatic hydrocarbons (or sometimes called arenes or aryl hydrocarbon) are hydrocarbons with sigma bonds and delocalized π electrons between carbon atoms forming rings.
-
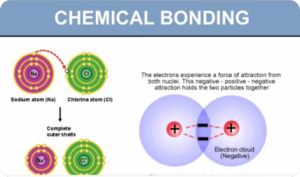
Chemical Bonding (Digital Notes)
$300.00 Add to cartChemical bonding is the interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up the familiar substances of the everyday world.
-

Chemical Energetics (Digital Notes)
$250.00 Add to cartThe study of chemical energetics deals with the energy changes during a chemical reaction. During chemical reactions, energy is either released or absorbed. Chemical energetics tells us whether a chemical reaction can take place spontaneously or not and about the feasibility of a reaction.
-
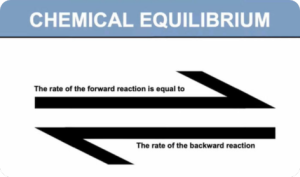
Chemical Equilibria (Digital Notes)
$100.00 Add to cartIn a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction.