Description
Course Contents
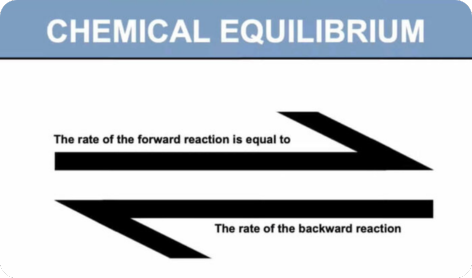
- Chemical equilibria: reversible reactions; dynamic equilibrium
- (i) factors affecting chemical equilibria
- (ii) equilibrium constants
- (iii) the Haber process
Learning Outcomes
Candidates should be able to:
- (a) explain, in terms of rates of the forward and reverse reactions, what is meant by a reversible reaction and dynamic equilibrium
- (b) state Le Chatelier’s Principle and apply it to deduce qualitatively (from appropriate information) the effects of changes in concentration, pressure or temperature, on a system at equilibrium
- (c) deduce whether changes in concentration, pressure or temperature or the presence of a catalyst affect the value of the equilibrium constant for a reaction
- (d) deduce expressions for equilibrium constants in terms of concentrations, Kc , and partial pressures, Kp [treatment of the relationship between Kp and Kc is not required]
- (e) calculate the values of equilibrium constants in terms of concentrations or partial pressures from appropriate data
- (f) calculate the quantities present at equilibrium, given appropriate data (such calculations will not require the solving of quadratic equations)
- (g) show understanding that the position of equilibrium is dependent on the standard Gibbs free energy change of reaction, ∆G⦵ [Quantitative treatment is not required]
- (h) describe and explain the conditions used in the Haber process, as an example of the importance of an understanding of chemical equilibrium in the chemical industry