Description
Course Contents
- Alcohols (exemplified by ethanol)
- (i) formation of halogenoalkanes
- (ii) reaction with sodium; oxidation; dehydration
- (iii) the tri-iodomethane test
- Phenol
- (i) its acidity; reaction with bases and sodium
- (ii) nitration of, and bromination of, the aromatic ring
Learning Outcomes
Candidates should be able to:
- (a) recall the chemistry of alcohols, exemplified by ethanol:
- (i) combustion
- (ii) nucleophilic substitution to give halogenoalkanes
- (iii) reaction with sodium
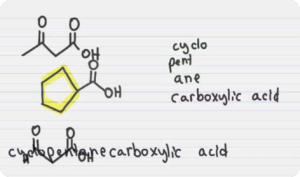
- (iv) oxidation to carbonyl compounds and carboxylic acids
- (v) dehydration to alkenes
- (b) suggest characteristic distinguishing reactions for the different classes of alcohols (primary, secondary and tertiary alcohols), e.g. mild oxidation
- (c) deduce the presence of a CH3CH(OH)– group in an alcohol from its reaction with alkaline aqueous iodine to form tri-iodomethane
- (d) recall the chemistry of phenol, as exemplified by the following reactions:
- (i) with bases
- (ii) with sodium
- (iii) nitration of, and bromination of, the benzene ring
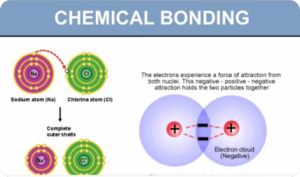
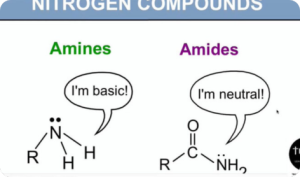
- (e) explain the relative acidities of water, phenol and ethanol in aqueous medium (interpret as BrønstedLowry acids)