Showing 13–17 of 17 results
-
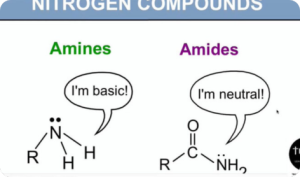
Nitrogen Compounds (Digital Notes)
$150.00 Add to cartNitrogen compounds such as urea, amine and guanidine are used to denature proteins, hence their antimicrobial properties. One special form of nitrogen compounds for antimicrobial treatment is the so-called quats or quaternary ammonium salts. These molecules have a positive charge, attracting the negatively charged cell membrane of microorganisms.
-
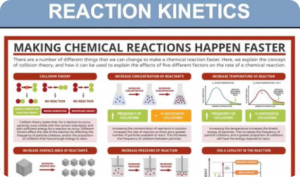
Reaction Kinetics (Digital Notes)
$250.00 Add to cartChemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction’s mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.
-

Stoichiometry and Redox (Digital Notes)
$150.00 Add to cartStoichiometry of redox reactions means calculations of the quantities of the oxidizing and reducing agents and their products in oxidation reduction reactions. Redox, on the other hand, is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, likewise, reduction is the gain of electrons or a decrease in the oxidation state.
-

The Gaseous State (Digital Notes)
$100.00 Add to cartThe gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases.
-
The Periodic Table (Digital Notes)
$100.00 Add to cartThe periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers.