Description
Course Contents
- Simple rate equations; orders of reaction; rate constants
- Concept of activation energy
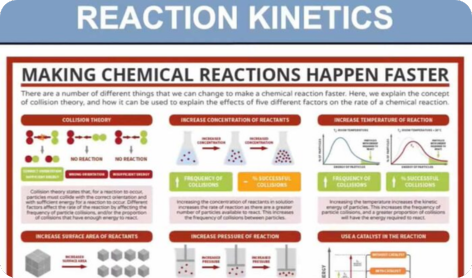
- Effect of concentration, temperature, and catalysts on reaction rate
- Homogeneous and heterogeneous catalysis
- Enzymes as biological catalysts
Learning Outcomes
Candidates should be able to:
- (a) explain and use the terms: rate of reaction; rate equation; order of reaction; rate constant; half-life of a reaction; rate-determining step; activation energy; catalysis
- (b) construct and use rate equations of the form rate = k[A]m[B]n (limited to simple cases of single-step reactions and of multi-step processes with a rate-determining step, for which m and n are 0, 1 or 2), including:
- (i) deducing the order of a reaction by the initial rates method
- (ii) justifying, for zero- and first-order reactions, the order of reaction from concentration-time graphs
- (iii) verifying that a suggested reaction mechanism is consistent with the observed kinetics
- (iv) predicting the order that would result from a given reaction mechanism
- (v) calculating an initial rate using concentration data [integrated forms of rate equations are not required]
- (c)
- (i) show understanding that the half-life of a first-order reaction is independent of concentration
- (ii) use the half-life of a first-order reaction in calculations
- (d) calculate a rate constant using the initial rates method
- (e) devise a suitable experimental technique for studying the rate of a reaction, from given information
- (f) explain qualitatively, in terms of frequency of collisions, the effect of concentration changes on the rate of a reaction
- (g) show understanding, including reference to the Boltzmann distribution, of what is meant by the term activation energy
- (h) explain qualitatively, in terms both of the Boltzmann distribution and of collision frequency, the effect of temperature change on a rate constant (and hence, on the rate) of a reaction
- (i)
- (i) explain that, in the presence of a catalyst, a reaction has a different mechanism, i.e. one of lower activation energy, giving a larger rate constant
- (ii) interpret this catalytic effect on a rate constant in terms of the Boltzmann distribution
- (j) outline the different modes of action of homogeneous and heterogeneous catalysis, including:
- (i) the Haber process
- (ii) the catalytic removal of oxides of nitrogen in the exhaust gases from car engines (see also Section 11.3)
- (iii) the catalytic role of atmospheric oxides of nitrogen in the oxidation of atmospheric sulfur dioxide
- (iv) catalytic role of Fe2+ in the I–/S2O82– reaction
- (k) describe enzymes as biological catalysts which may have specific activity
- (l) explain the relationship between substrate concentration and the rate of an enzyme-catalysed reaction in biochemical systems